Know the Biology, Accelerate the Cure
Amprion partners with leading pharmaceutical companies to advance the development of therapies for neurodegenerative diseases. Our patented seed amplification assay (SAA) technology provides unprecedented insight into the biology of synucleinopathies and other protein misfolding disorders, enabling more efficient and targeted drug development.
We have the real possibility to impact the life of millions and millions of people if we can identify the disease before it damages the brain.
The SAA Advantage for Your Trials
Incorporating Amprion’s seed amplification assays (SAA) into your development program isn't just a scientific upgrade—it's a strategic imperative for de-risking trials, accelerating timelines, and ensuring that your therapeutic asset stands out in an increasingly competitive and precision-driven marketplace. By leveraging this biomarker, sponsors can streamline development, validate MOA, support regulatory submission, and sustain long-term clinical value.

Accelerate.
Accelerate Development with Pathology-Proven Biomarkers
Traditional neurodegenerative trials face challenges due to slow biological progression and clinical variability. Amprion’s CSF α-synuclein SAA provides a direct and early measure of disease pathology, empowering faster, data-driven decisions throughout drug development.
The Amprion Advantage: Rapid, In Vitro Validation of Drug-Target Engagement
- Models in vivo pathology in a compressed timeline to assess seeding activity in vitro.
- Informs on mechanism of action (MOA) in preclinical and clinical stages—vital for α-syn-targeting agents like immunotherapies, ASOs, or aggregation inhibitors.
- Supports longitudinal biomarker tracking, revealing biological response before clinical symptoms appear.
Claim the Advantage
- Accelerate go/no-go decisions with clear early indicators of biological activity
- Accelerate go/no-go decisions with clear early indicators of biological activity
- Unlock novel development pathways with mechanistic confidence grounded in pathology

De-risk.
De-risk Your Trials with Precision Enrollment
Clinical phenotypes alone are insufficient to confirm underlying synuclein pathology, leading to heterogeneous trial populations and inconsistent efficacy signals.
The Amprion Advantage: Pathology-based Patient Selection
- Leverage Amprion’s seed amplification assays (SAAs) to confirm the presence or absence of pathogenic misfolded proteins, ensuring inclusion of participants with a verified synucleinopathy (e.g., PD, DLB, MSA).
- Identify patients in prodromal stages, such as RBD or MCI, with positive CSF synSAA, for early intervention trials.
- Reduce diagnostic uncertainty and eliminate off-target enrollment, a key factor in trial failures.
Claim the Advantage
- Improve trial homogeneity
- Maximize the likelihood of treatment effect detection
- Reduce the risk of underpowered studies

Differentiate.
Differentiate Regulatory and Market Positioning with Pathology Confirmation
Regulatory agencies increasingly expect biological confirmation of disease for trial inclusion and approval in therapeutic development programs for neurodegenerative disorders.
The Amprion Advantage: Pathology-Aligned Development Strategy
- Aligns with the FDA and EMA’s evolving biomarker qualification efforts and trends in precision neurology.
- Offers a validated diagnostic tool to support enrichment strategies or label expansion.
- Facilitates clear differentiation in a crowded pipeline by demonstrating mechanism-based efficacy in pathology-confirmed populations.
Claim the Advantage
- Increase regulatory confidence
- Support the use of accelerated approval pathways
- Improve payer and provider confidence in clinical utility
The α-syn SAAs produce consistently robust results in sensitivity and specificity in identifying patients with PD compared to healthy controls and other non-synuclein neurological disorders, and can be measured reliably in at-risk individuals several years prior to onset of clinical symptoms. Assessment of α-syn by SAA represents a methodology that may enable the development of interventions for Parkinson’s disease and biologically related conditions prior to onset of clinical manifestations.
Your Partner in Precision
Medicine for Synucleinopathies
Amprion empowers global biopharma studies and clinical trials in neurodegenerative diseases by providing highly accurate, early biomarker detection and comprehensive insights to accelerate drug discovery and improve patient outcomes.
Amprion operates the world’s first and only US CAP/CLIA-certified laboratory offering the validated SAAmplify-αSYN test as a Laboratory Developed Test (LDT), commercially available for clinical and research use.
The US FDA has granted Breakthrough Device Designation to Amprion and issued a Letter of Support recommending the use of α-syn SAA for research and clinical trials, underscoring its scientific credibility and clinical value.
Scientific Leadership and Innovation
- Amprion’s scientific leadership has pioneered seed amplification methodologies, which are now recognized as foundational in the field of neurodegenerative disease diagnostics and reflected in numerous peer-reviewed publications validating the clinical utility of their assays.
- The company holds multiple U.S. and international patents, demonstrating ongoing innovation and commitment to advancing biomarker science
Proven Clinical Accuracy and Validation
- The SAAmplify-αSYN CSF test demonstrates 96% sensitivity and 92% specificity for α-synuclein pathology, delivering autopsy-confirmed accuracy that is unmatched in the field.
- The test is clinically validated, performed in a CAP-accredited, CLIA-certified laboratory, and has received FDA Breakthrough Device Designation for detecting misfolded α-synuclein.
Accelerates Precision Medicine and Drug Development
- Amprion’s technology enables early and accurate identification of disease pathology, supporting patient stratification, endpoint selection, and monitoring in clinical trials, which is critical for developing targeted therapies in neurodegenerative diseases.
- The platform helps biopharma partners identify new drug candidates and underlying pathologies, including in cases where traditional endpoints may have failed, thus optimizing trial design and outcomes.
Integrated Diagnostic Services and Data Management Solutions
- Amprion’s project teams coordinate sample logistics, results reporting, and data management across clinical sites, sponsors, and investigators, ensuring smooth study execution
- Data services include secure data handling, robust analytics, and customized reporting, supporting regulatory compliance and facilitating decision-making for trial sponsors and biopharma partners.
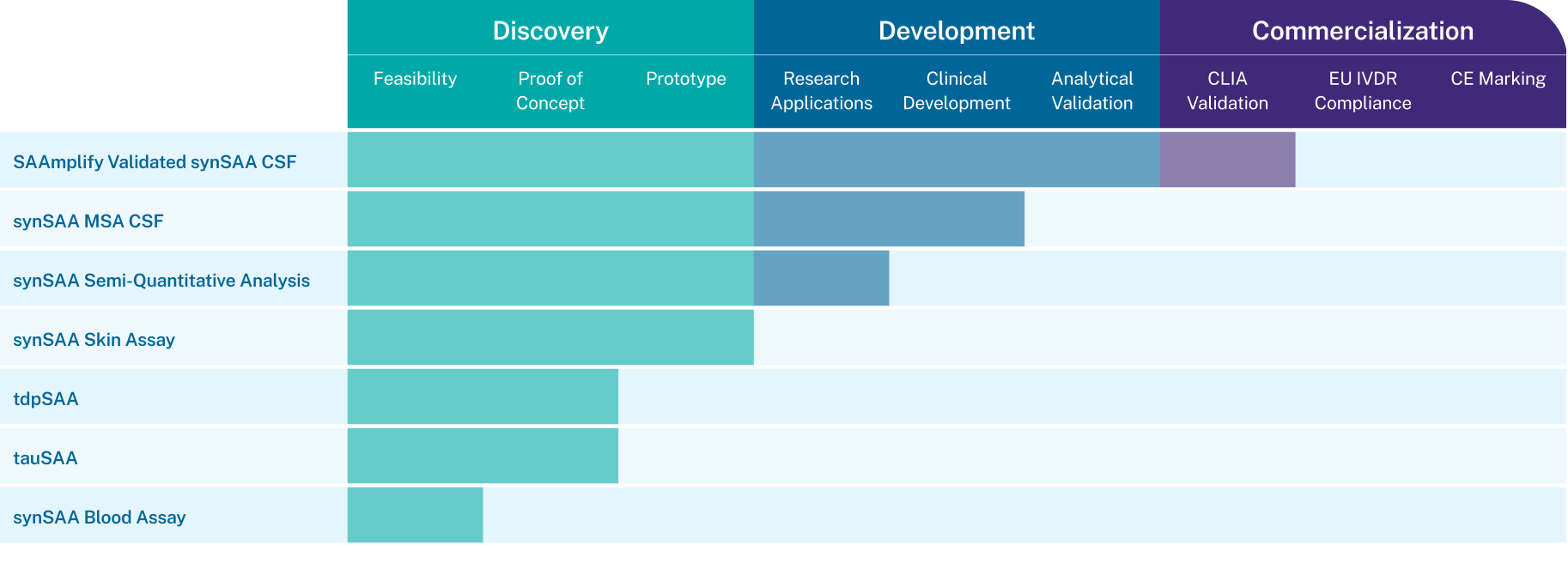
Amprion’s Innovation Pipeline Fuels Therapeutic Development
Peer-Reviewed Proof, Not Just Claims
While others claim accuracy, we back it up with proof: Our technology is supported by 400+ autopsy confirmations, 1,200+ scientific publications, and analysis of over 20,000 samples. Our evidence-based approach delivers certainty where it matters most.
Scientific Paper
α-Synuclein Seed Amplifications Assay in a Cohort With Cognitive Impairment Performance and Interactions With CSF and Plasma Biomarkers
Scientific Paper
Longitudinal decline in striatal DAT binding in LRRK2 Parkinson's disease: connections with CSF α-synuclein seeding activity

Scientific Paper
Prodromal Lewy Body Symptoms and α-Synuclein Seeding in Idiopathic Olfactory Dysfunction
