Lewy Body Pathology and APOE ε4 Shape ARIA Risk in Alzheimer’s Disease: A New Framework for Patient Selection in Anti-Amyloid Therapy
Anti-amyloid monoclonal antibodies such as lecanemab represent a major advance for patients with Alzheimer’s disease (AD). Yet, their use is complicated by the risk of edema and hemorrhages in the brain, which can be detected radiographically by MRI as amyloid-related imaging abnormalities (ARIA). ARIA is a major cause of therapeutic discontinuation.
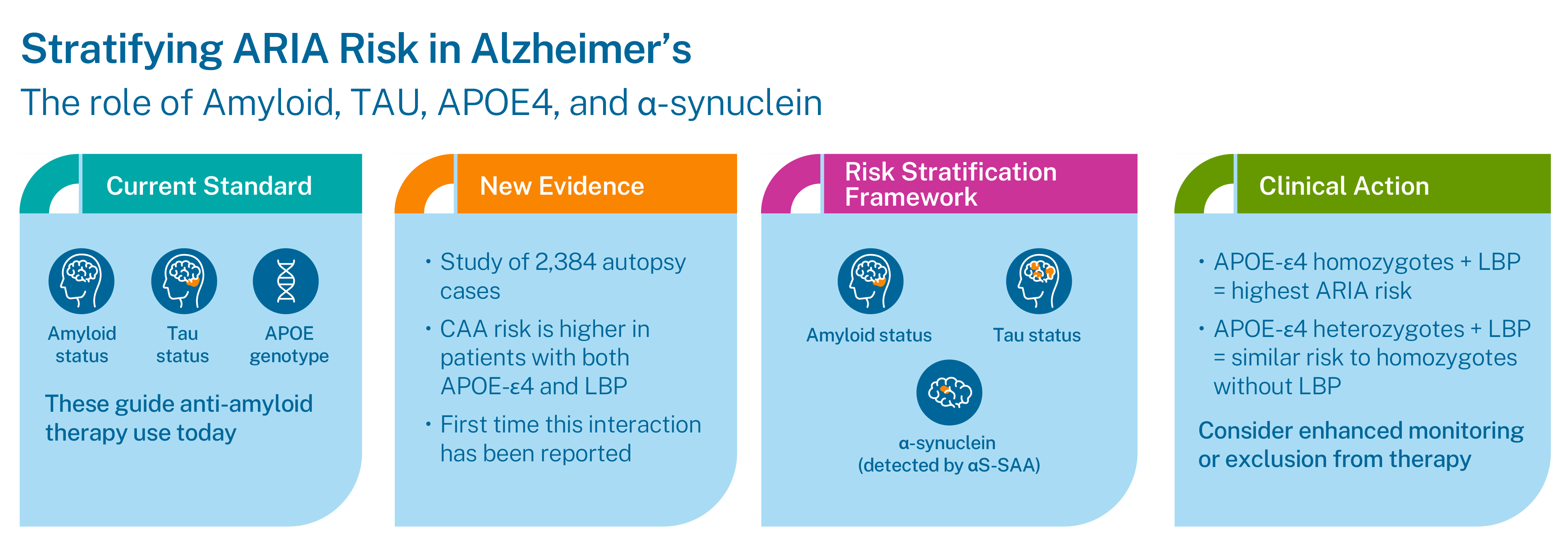
A recent study by Pillai et al. (2024) analyzed over 2,300 autopsy-confirmed dementia cases to clarify how Lewy body pathology (LBP) modifies risk factors for cerebral amyloid angiopathy (CAA), the key pathological correlate of ARIA.
Key Findings of the Study
- APOE ε4 and LBP act synergistically to increase CAA risk.
While APOE ε4 homozygosity is already a strong risk factor for CAA and ARIA, APOε4 heterozygosity with LBP carries comparable risk factors.
- APOE ε4 heterozygotes with LBP also carry significantly increased risk of CAA.
- Presumably, the increased frequency of CAA in these patients will translate to heightened risk for ARIA, although this was not directly assessed in this postmortem study.
- This is the first report showing that LBP reshapes the APOE risk curve.
- Novelty: The link between CAA, APOE ε4, and LBP has not been reported in prior autopsy series.
- Direct quote: “The development of aggregated alpha-synuclein biomarker methods to detect co-existent LBP in patients with AD may be necessary to fully characterize underlying neuropathology in mild cognitive impairment or dementia patients being evaluated for amyloid therapies.”
- LBP detection matters. CSF α-synuclein seed amplification assays (αS-SAA) represent the most accurate biomarker to detect α-synuclein aggregates during life, which correlates with the presence of LBP at autopsy.
Clinical Perspective: Rethinking Anti-Amyloid Therapy Risk Stratification
Today’s standard approach to anti-amyloid therapy candidacy focuses on amyloid and tau positivity plus APOE ε4 genotype. However, this study suggests we need to add a fourth dimension: Lewy body pathology. This addition may improve predictions of both treatment responsiveness and risk for serious adverse events.
- Pre-treatment evaluation should include:
- Amyloid status (PET imaging or CSF/plasma Aβ42/Aβ40)
- Tau status (CSF/plasma p-Tau217, PET imaging)
- α-Synuclein aggregates (CSF αS-SAA)
- APOE ε4 genotype
- Risk implications:
- APOE ε4 homozygotes with LBP → highest risk for CAA and ARIA.
- APOE ε4 heterozygotes with LBP → risk comparable to homozygotes without LBP
These patients may require similar cautious use of anti-amyloid therapies and very careful monitoring of ARIA.
Clinical Takeaway: A Precision Framework for ARIA Risk Stratification
This emerging framework supports a precision medicine approach to anti-amyloid therapy. Before initiating treatment, clinicians should consider integrating a comprehensive biomarker panel consisting of amyloid, tau, APOE ε4, and α-synuclein (via CSF αS-SAA) to stratify ARIA risk.
By stratifying patients using this four-dimensional approach, clinicians can:
- Proactively mitigate risk by identifying those most vulnerable to ARIA
- Tailor monitoring protocols for higher-risk individuals
- Potentially avoid preventable adverse events, particularly in patients with combined APOE ε4 carriage and LBP

Get in the Know
Bring biological certainty to your diagnosis with Amprion's SAAmplify-ɑSYN test. Order your shipping kits today and help move your patient beyond a probable diagnosis.
